Pablo Artigas, Ph.D.
Professor of Cell Physiology & Molecular Biophysics

Department of Cell Physiology and Molecular Biophysics
Texas Tech University Health Sciences Center
3601 4th Street, STOP 6551
Lubbock, Texas 79430
Phone: (806) 743-1136
FAX: (806) 743-1512
Research Interest:
Our research focuses on understanding the function, mechanisms and pharmacology
of the proteins that trans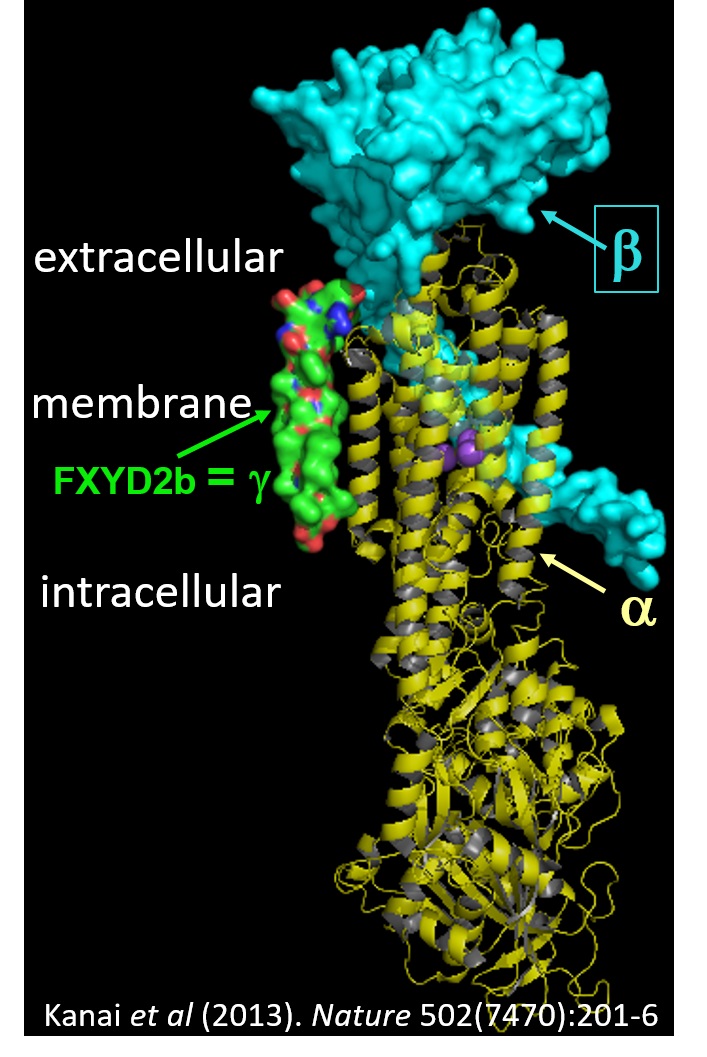 port ions across membranes. In particular, we study how the sodium/potassium (Na/K)
and proton/potassium (H/K) pumps operate at the molecular level, as well as their
role in health and disease. These two P-type-II ATPases couple ATP hydrolysis to ion
transport against their electrochemical gradients. The Na/K pump extrudes 3 Na+ in exchange for 2 K+, while the H/K pump hydrolyses ATP to export H+ and import K+. Both pump types are formed by association of a catalytic α- and an auxiliary β-subunit,
which are frequently associated with a FXYD regulatory subunit, as shown in the figure.
Several genes encode for different isoforms that form distinct Na/K and H/K pump enzymes,
which are expressed with a tissue specific distribution.
The gastric H/K pump acidifies the stomach and is the
target of the antacid drug omeprazole. The non-gastric H/K pump participates in K+ reabsorption and is important in cystic fibrosis pathology. The Na/K pump is present
in all human cells. Mutations to the Na/K pump α1 subunit lead to Conn’s syndrome
(primary aldosteronism), Charcot-Marie-Tooth disease type 2 and hypomagnesemia with
seizures, mutation to the Na/K pump α2 lead to migraine, and mutation to Na/K pump
α3 lead to alternating hemiplegia of childhood and Parkinsonism.
port ions across membranes. In particular, we study how the sodium/potassium (Na/K)
and proton/potassium (H/K) pumps operate at the molecular level, as well as their
role in health and disease. These two P-type-II ATPases couple ATP hydrolysis to ion
transport against their electrochemical gradients. The Na/K pump extrudes 3 Na+ in exchange for 2 K+, while the H/K pump hydrolyses ATP to export H+ and import K+. Both pump types are formed by association of a catalytic α- and an auxiliary β-subunit,
which are frequently associated with a FXYD regulatory subunit, as shown in the figure.
Several genes encode for different isoforms that form distinct Na/K and H/K pump enzymes,
which are expressed with a tissue specific distribution.
The gastric H/K pump acidifies the stomach and is the
target of the antacid drug omeprazole. The non-gastric H/K pump participates in K+ reabsorption and is important in cystic fibrosis pathology. The Na/K pump is present
in all human cells. Mutations to the Na/K pump α1 subunit lead to Conn’s syndrome
(primary aldosteronism), Charcot-Marie-Tooth disease type 2 and hypomagnesemia with
seizures, mutation to the Na/K pump α2 lead to migraine, and mutation to Na/K pump
α3 lead to alternating hemiplegia of childhood and Parkinsonism.
Ongoing Projects:
ROLE OF NA/K PUMPS AND OTHER ION-TRANSPORT MECHANISMS IN ADAPTATION TO EXTREME SALINE ENVIRONMENTS
The brine shrimp (crustaceans of the order Anostraca, genus Artemia) and brine fly larvae are the only two animals known to live in the Great Salt Lake of Utah at ~ 14% salinity (about four times that of seawater). Brine shrimp, which can survive in salinities up to 10 times of that in normal sea water, are known to have two α subunits for the Na/K pump. One of these α subunits has several modifications around the ion-binding sites, which make it very different to most Na/K pumps found in other animals. Teleost fish present similar modifications, but when adapting to fresh water rather than high salinity. We are trying to uncover how the Na/K pump interacts with other ion transport mechanisms to provide adaptive advantage to extremely high salinities, as well as the functional importance of the functional modifications induced special Na/K pump versions to the animals that carry them. In this project, we use transcriptome analysis and several other molecular biology techniques, immunostaining, radioactive isotope uptake, heterologous protein expression in oocytes, and electrophysiology.
MECHANISM OF DISEASE INDUCTION BY NA/K PUMP α1 MUTATIONS.
We aim to understand how mutation of the pump’s α1 subunit lead to disease. We have demonstrated that several Na/K pump mutations found in aldosterone-producing adenomas (benign tumors of the adrenal gland) from patients with primary aldosteronism (Conn’s syndrome) lead to loss-of-function (Meyer et al, 2017, 2019). We are now using transgenic mice, as well as heterologous expression and electrophysiological analysis in Xenopus oocytes, of mutations inducing CMT or hypomagnesemia and seizures, to uncover the pathophysiologic mechanisms of these Na/K pump-related diseases.
UNCOVERING ION SELECTIVITY, REGULATION AND PHARMACOLOGY NA/K AND H/K PUMPS
It remains unclear how the Na/K pump selects Na+ over the more abundant K+ when facing the inside of the cell, or how it selects K+ over Na+ when facing the external milieu. It is also unclear what determines the distinct ion selectivity and regulation of Na/K and H/K pumps. We are currently using a combination of mutagenesis (including incorporation of unnatural amino acids), voltage clamp fluorometry, patch clamp and molecular dynamics (in collaboration with Benoît Roux) to untangle the mechanisms of ion selectivity at the atomic level. From a translational science point of view, we are trying to develop new drugs to selectively inhibit or modulate different H/K pumps in order to treat disease.
Techniques:
We use an array of techniques that include electrophysiology (patch-clamp and two-electrode voltage clamp) molecular biology (transcriptome analysis, site-directed mutagenesis, non-sense suppression, heterologous protein expression and western blot), biochemistry (ATPase activity and radiolabeled ion uptake measurements), fluorescence microscopy (voltage clamp fluorometry, immunocytochemistry) and mouse behavioral analysis to study the role of the mechanisms of Na/K pump function and their role in physiology and pathophysiology.
Funding:
We are thankful to all agencies (federal and private) that have supported our research.
Active: National Institute of Health, National Science Foundation, and the CH Foundation
Past: National Science Foundation, National Institute of Health, CH Foundation, American
Heart Association, South Plains Foundation, and Laura Bush Institute for Women's Health.
Lab Members
Kerri Spontarelli
Daniel Self
Joseph Eleruja
Navya Kavuri
Arnav Gaitonde
Collaborators
Professor, Department of Molecular Physiology and Biophysics, University of Iowa
Professor, Director, School of Biological Sciences, Illinois State University
Professor, Department of Cell and Molecular Physiology, Stritch School of Medicine, Layola University
Professor, Department of Biochemistry and Molecular Biophysics, University of Chicago
Professor, Department of Biomedicine, Aarhus University, Denmark
Got Questions?
We're here to help. Contact us if you have questions.
