Ina L. Urbatsch, Ph.D.
Professor

Ph.D. Chemistry/Biochemistry
University of Kaiserslautern, Germany
Curriculum Vitae
Department of Cell Biology and Biochemistry
Texas Tech University Health Sciences Center
3601 4th Street, Lubbock, TX 79430-6540
Office Phone: (806) 743-1192
ina.urbatsch@ttuhsc.edu
Research Interests
ABC Transporters in Cancers, Cystic Fibrosis and Atherosclerosis
Current Projects
ABC transporter Superfamily:
The ATP-binding cassette (ABC) transporter superfamily comprises a large number of
membrane spanning transport proteins that extends from bacteria to man. They are associated
with many human disorders, including cystic fibrosis, immunodeficiency, retinal degeneration,
and defects in lipid and cholesterol metabolic pathways. In addition, several transporters
are involved in cancer drug resistance, resulting in poor treatment outcome and increased
patient relapse. ABC transporter typically consist of two transmembrane domains (TMDs)
and two nucleotide-binding domains (NBDs). The TMDs provide the transport pathway
for a particular substrate, and the NBDs power the transport by ATP hydrolysis. Consistent
with their functional diversity (e.g., transport of ions, peptides, hydrophobic drugs,
lipids, cholesterol) the transmembrane domains exhibit weak similarity, whereas the
NBDs are highly conserved. We recently crystallized P-glycoprotein (Pgp), an ABC transporter
involved in multidrug resistance of cancers. In this nucleotide-free structure the
NBDs are ~30 Å apart, exposing a central cavity formed by the TMDs to the inner leaflet
of the membrane and the cytoplasm. Co-crystal structures of Pgp with the cyclopeptide
inhibitors have located these inhibitor binding sites in the center of the membrane
bilayer (Fig. 1A). Comparison of nucleotide-free Pgp with nucleotide-bound structures
of other ABC transporters led to an alternating-access model, where the central cavity
is accessible to only one side of the membrane at a time. In this model, Pgp switches
between inward- and outward-facing conformations, gated by ATP binding, resulting
in NBD dimerization and ATP hydrolysis that then reopens the NBD dimer. Still, many
questions remain about the sequence of conformational changes (simplified as a two-step
oscillation in Fig. 1) leading to the NBD dimerization and reopening of the dimer,
or the mechanisms by which ATP hydrolysis is coupled to substrate translocation.
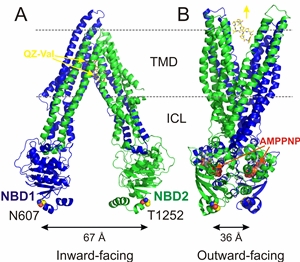
Figure 1: X-ray structures of nucleotide-free Pgp and nucleotide-bound MsbA. In the inward-facing Pgp structure (left, 4Q9J), the NBDs are distant from each other, and two bundles of transmembrane helices surround a drug binding cavity that is open to the inner membrane leaflet and cytoplasm. N- and C-terminal Pgp halves (blue and green), two QZ-Val molecules (gray sticks), and residues F332, L335, N607 and T1256 (yellow spheres) are shown. In the outward-facing AMPPNP-bound MsbA structure (right, 3B5Y) the TM helices have rotated and opened to the outer leaflet. ICLs: intracellular loops; gray dashed lines denote the approximate boundaries of the membrane.
Szewczyk P, Tao H, McGrath AP, Villaluz M, Rees SD, Lee SC, Doshi R, Urbatsch IL, Zhang Q and Chang G. Snapshots of Ligand Entry, Malleable Binding, and Induced Helical Movement in P-glycoprotein. Acta Crystallographica Section D, 2015; 71(Pt 3):732-41.
Moeller A, Lee SC, Tao H, Speir JA, Chang G, Urbatsch IL, Potter CS, Carragher B, and Zhang Q. Distinct Conformational Spectrum of Homologous Multidrug ABC Transporters. Structure, 2015;
Role of P-glycoprotein (Pgp) in multidrug resistance of cancers:
Breast cancer is a major health problem with one million new cases annually and roughly
370,000 deaths worldwide. Often chemotherapy fails because the patient’s tumors either
were resistant at the outset of treatment or eventually develop resistance after exposure
to the cancer drugs. Several other ABC transporters such as the Breast Cancer Resistance
Protein (BCRP) and the Multidrug Resistance Associated Proteins (MRP) have been implicated
in drug resistance. Yet, Pgp is the most prevalent and best-studied cause of multidrug
resistance. Its expression in tumors is associated with poor treatment outcome and
increased patient relapse. This has made Pgp a therapeutic target since its discovery
more than three decades ago. Besides its relevance to cancer treatment, the pharmaceutical
industry has also targeted Pgp for its role in MDR of HIV, epilepsy and psychiatric
illnesses. Several inhibitors of Pgp have been tested in clinical trials, but none
have been approved for clinical use because of their toxicities and unfavorable interactions
with organs involved in drug clearance. Consequently, there is currently great interest
in understanding the mechanism by which drugs interact with Pgp, with the aim of developing
inhibitors that can be used in combination with cancer drugs to improve the response
of tumors to chemotherapeutic agents. For this purpose a thorough understanding of
drug/inhibitor interactions with Pgp is essential.
Role of CFTR in Cystic Fibrosis:
Cystic Fibrosis (CF) is perhaps the most common fatal genetic disease in the Western
world. CF results from deficiencies in the chloride ion transport of epithelial cells
that line the lungs, intestines, and other organs. In the lungs this leads to accumulation
of thick dehydrated mucus and eventually permanent lung damage and death. Presently,
only the symptoms of CF can be treated, and although the average life expectancy for
CF patients has improved dramatically to ~40 years, there is no cure yet. CF is caused
by mutations in the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene.
This gene encodes an ABC transporter with two non-identical nucleotide binding domains
(NBDs) and two transmembrane domains that harbor the chloride ion channel pore. Opening
and closing of the channel are controlled by ATP-driven events at the NBDs and are
somehow strictly regulated by phosphorylation of a regulatory (R-) domain that is
specific to CFTR. Much remains to be learned about the interactions between the two
NBDs, the channel pore, and the R-domain, and how they regulate gating. Such knowledge
may provide insight into strategies for increasing channel activity, and thus for
modulating ion flux and water movement in cases of disease. The critical enabling
information to study assembly and function at the molecular level is the production
of purified, highly active protein is necessary in sufficient quantities for biophysical
studies.
Role of ABCG5 and ABCG8 in Sterol and Cholesterol Transport:
Hypercholesterolemia is a major risk factor for cardiovascular disease. It develops
from an imbalance in cholesterol homeostasis, a finely tuned process involving many
steps including de novo synthesis, absorption from the diet, trafficking through the
body via lipoproteins and excretion through the liver and intestines. Sitosterolemia
is a hypercholesterolemia-like disease characterized by significantly elevated plasma
levels of plant and shellfish sterols. This genetic disorder also causes hyperabsorption
and insufficient excretion of cholesterol, although plasma levels appear normal in
half of the cases because de novo biosynthesis of cholesterol is downregulated. The
clinical syndromes include the development of tendon and tuberous xanthomas and premature
coronary arteriosclerosis due to significantly increased absorption from the diet
and decreased excretion of sterols into bile. Sitosterolemia is caused by mutations
in the ABCG5 and ABCG8 genes, suggesting that these gene products play a role in the
efficient removal of plant sterols and cholesterol (Fig. 2) and thus, are directly
involved in the regulation of cholesterol homeostasis.
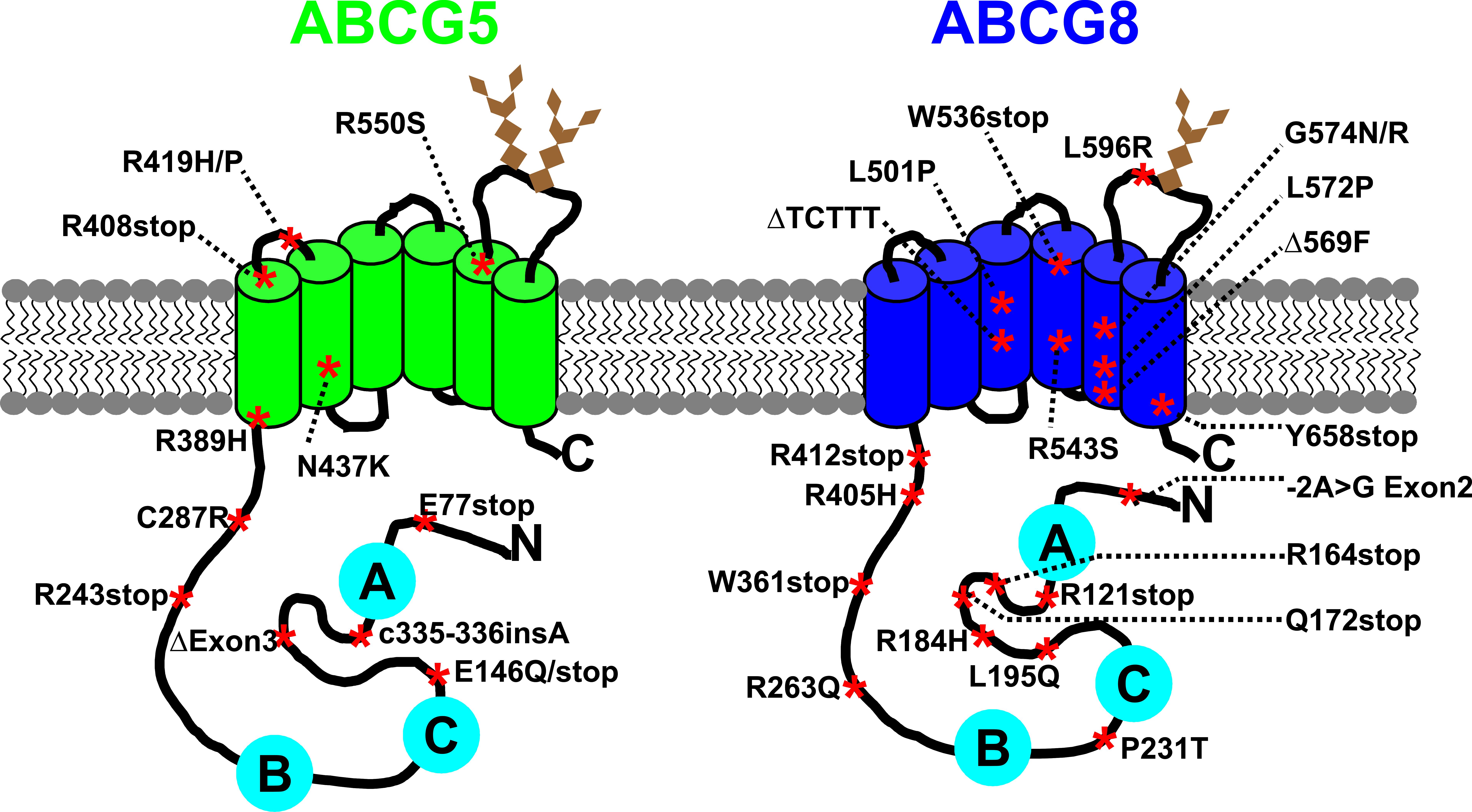
Figure 2: Putative topology of ABCG5 and ABCG8 with mutations known to cause sitosterolemia. ABCG5 and ABCG8 are half-size ABC transporter each containing six transmembrane -helices (green and blue cylinders) and a cytoplasmic nucleotide binding domain (NBD). Mutations that cause sitosterolemia are indicated by a red asterisk (stop, premature termination; ins, insertion; Δ, deletion). The conserved Walker A, Walker B, and C motifs are shown in cyan circles, and N-glycosylation sites in brown diamonds. Based on Swiss-Prot entries Q9H222 and Q9H221.
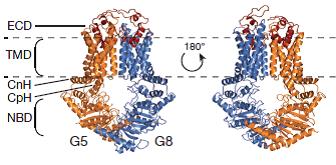
Figure 3: Crystal structure of ABCG5/ABCG8 solved in lipid bicelles. The structure represents a new fold typical for ABCG subfamily members.
Lee JY, Kinch LN, Borek DM, Wang J, Wang J, Urbatsch IL, Xie XS, Grishin NV, Cohen JC, Otwinowski Z, Hobbs HH, Rosenbaum DM. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature. 2016 May 4;533(7604):561-4. doi: 10.1038/nature17666.
Johnson BJ, Lee JY, Pickert A, Urbatsch IL. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry. 2010; 49(16):3403-11.
The biomechanics of the sterol transport mechanism by ABCG5/G8 is still largely unknown, but can be elucidated from studies of their biochemical properties and their molecular structures. Such information is critical to understand how ABCG5 and ABCG8 regulate cholesterol in the body, and will provide insights into strategies for modulating sterol uptake and excretion in cases of sitosterolemia and more importantly, arteriosclerosis.
Selected Publications
(From 79 total publications, H Index = 35)
Full publications list: NCBI My Bibliography Google Scholar
- Gewering T, Waghray D, Parey K, Jung H, Tran NNB, Zapata J, Zhao P, Chen H, Januliene D, Hummer G, Urbatsch I, Moeller A, Zhang Q. Tracing the substrate translocation mechanism in P-glycoprotein. Elife. 2024 Jan 23;12. doi: 10.7554/eLife.90174. PMID: 38259172 PubMed
- Tran NNB, Bui ATA, Jaramillo-Martinez V, Weber J, Zhang Q, Urbatsch IL. Lipid environment determines the drug-stimulated ATPase activity of P-glycoprotein. Front Mol Biosci. 2023 Feb 23; 10: 1141081. doi: https://doi.org/10.3389/fmolb.2023.1141081. eCollection 2023, PMID: 3691152 PubMed
- Wang C, Yang Z, Loughlin BJ, Xu H, Veit G, Vorobiev S, Clarke OB, Jiang F, Li Y, Singh S, Rich Z, Menten ER, Grassucci RA, Wang W, Mezzell A, Fu Z, Wong KH, Wang J, Wetmore DR, Sutton RB, Brouillette CG, Urbatsch IL, Kappes JC, Lukacs GL, Frank J, Hunt JF, Mechanism of dual pharmacological correction and potentiation of human CFTR, preprinted in bioRxiv Oct 11, 2022; doi: https://doi.org/10.1101/2022.10.10.510913
- Gewering T, Waghray D, Parey K, Zapata J, Zhao P, Chen H, Januliene D, Urbatsch IL, Moeller A, Zhang Z, Mechanism of multimodal substrate translocation in P-glycoprotein, posted in bioRxiv April 29, 2022. doi: https://doi.org/10.1101/2022.04.28.489897
- Jaramillo Martinez V, Ganapathy V, Urbatsch IL (2022). Peptide Tags and Domains for Expression and Detection of Mammalian Membrane Proteins at the Cell Surface. Methods Mol Biol. 2022; 2507:337-358. doi: 10.1007/978-1-0716-2368-8_18. PMID: 35773591 PubMed
- Jaramillo-Martinez V, Sivaprakasam S, Ganapathy V, Urbatsch IL. Drosophila INDY and Mammalian INDY: Major Differences in Transport Mechanism and Structural Features despite Mostly Similar Biological Functions. Metabolites. 2021 Sep 29;11(10):669. doi: 10.3390/metabo11100669. PMID: 34677384 PubMed
- Jaramillo-Martinez V, Ganapathy V, Urbatsch IL. A home run for human NaCT/SLC13A5/INDY: cryo-EM structure and homology model to predict transport mechanisms, inhibitor interactions and mutational defects. Biochem J. 2021 Jun 11; 478(11): 2051-2057. doi: 10.1042/BCJ20210211. PubMed
- Jaramillo-Martinez V, Urbatsch IL, Ganapathy V. (Urbatsch is co-corresponding author). Functional Distinction between Human and Mouse Sodium-Coupled Citrate Transporters and Its Biologic Significance: An Attempt for Structural Basis Using a Homology Modeling Approach. Chem Rev. 2021 May 12; 121(9): 5359-5377. doi: 10.1021/acs.chemrev.0c00529. Epub 2020 Oct 11. Chem Reviews has a Thomson Reuters’ Web of Science impact factor of 54. PubMed
- Higuchi K, Kopel JJ, Sivaprakasam S, Jaramillo-Martinez V, Sutton RB, Urbatsch IL, Ganapathy V. Functional analysis of a species-specific inhibitor selective for human Na+-coupled citrate transporter (NaCT/SLC13A5/mINDY). Biochem J. 2020 Nov 13; 477(21):4149-4165. PubMed
- Swartz DJ, Singh A, Sok N, Thomas JN, Weber J, Urbatsch IL. Replacing the Eleven Native Tryptophans by Directed Evolution Produces an Active P-glycoprotein With Site-Specific, Non-Conservative Substitution. Sci Rep. 2020 Feb 21; 10(1): 3224. PubMed
- Sigoillot M, Overtus M, Grodecka M, Scholl D, Garcia-Pino A, Laeremans T, He L, Pardon E, Hildebrandt E, Urbatsch IL, Steyaert J, Riordan JR and Govaerts C. Domain-interface Dynamics of CFTR Revealed by Stabilizing Nanobodies. Nature Communications 2019 June; 10(1): 2636 2019. PubMed
- Yang Z, Hildebrandt E, Jiang F, Aleksandrov AA, Khazanov N, Zhou Q, An J, Mezzell AT, Xavier B, Ding H, Riordan JR, Senderowitz H, Kappes JC, Brouillette C G, and Urbatsch IL. Structural stability of purified human CFTR is systematically improved by mutations in nucleotide binding domain 1. Biochimica et Biophysica Acta 2018 May; 1860(5): 1193-1204. doi: 10.1016/j.bbamem.2018.02.006. PubMed
- Zoghbi ME, Mok L, Swartz DJ, Singh A, Fendley G, Urbatsch IL, and Altenberg GA. Substrate binding modulates the conformational changes of the nucleotide binding domains of the multidrug transporter P-glycoprotein in a lipid bilayer. J. Biol. Chem. 2017; 292(50), 20412-20424. Urbatsch is co-corresponding author. PubMed
- Xavier BM, Hildebrandt E, Jiang F, Ding H, Kappes JC, and Urbatsch IL. Substitution of Yor1p NBD1 residues improves the thermal stability of Human Cystic Fibrosis Transmembrane Conductance Regulator, Protein Engineering, Design and Selection 2017 Oct 1; 30(10): 729-741. PubMed
- Hildebrandt E, Khazanov N, Kappes J C, Dai Q, Senderowitz H, Urbatsch IL, (2017). Specific stabilization of CFTR by phosphatidylserine. Biochimica et biophysica acta, 1859(2), 289-293. PubMed
- Yang Z, Zhou Q, Mok L, Singh A, Swartz DJ, Urbatsch IL, Brouillette CG, (2017). Interactions and cooperativity between P-glycoprotein structural domains determined by thermal unfolding provides insights into its solution structure and function. Biochimica et biophysica acta, 1859(1), 48-60. PubMed
- Lee JY, Kinch LN, Borek DM, Wang J, Wang J, Urbatsch IL, Xie XS, Grishin NV, Cohen JC, Otwinowski Z, Hobbs HH, Rosenbaum DM. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature. 2016 May 4;533(7604):561-4. doi: 10.1038/nature17666. PubMed
- Szewczyk P, Tao H, McGrath AP, Villaluz M, Rees SD, Lee SC, Doshi R, Urbatsch IL, Zhang Q and Chang G. Snapshots of Ligand Entry, Malleable Binding, and Induced Helical Movement in P-glycoprotein. Acta Crystallographica Section D, 2015; 71(Pt 3):732-41. PMCID: PMC4356375. the article has already been cited 7 times in Thomson Reuters’ ISI Web of Knowledge. PubMed
- Moeller A, Lee SC, Tao H, Speir JA, Chang G, Urbatsch IL, Potter CS, Carragher B, and Zhang Q. Distinct Conformational Spectrum of Homologous Multidrug ABC Transporters. Structure, 2015; 23(3):450-60. PMCID: PMC4351144 Structure has a high impact factor of 6.8 in Thomson Reuters’ ISI Web of Knowledge. PubMed
- Hildebrandt E, Mulky A, Ding H, Dai Q, Aleksandrov AA, Bajrami B, Diego PA, Wu X, Ray M, Naren AP, Riordan RR, Yao X, DeLucas LJ, Urbatsch IL, and Kappes JC (co-corresponding author). A Stable Human-Cell System Overexpressing Cystic Fibrosis Transmembrane Conductance Regulator Recombinant Protein at the Cell Surface. Mol Biotechnol. 2015 May;57(5):391-405. PMCID: PMC4405497 PubMed
- Hildebrandt E, Zhang Q, Cant N, Ding H, Dai Q, Peng L, Fu Y, DeLucase LJ, Ford R, Kappes JF, and Urbatsch IL. A survey of detergents for the purification of stable, active human cystic fibrosis transmembrane conductance regulator (CFTR). Biochim Biophys Acta. 2014 Jul 24;1838(11):2825-2837. PMCID: PMC4170525 PubMed
- Swartz DJ, Mok L, Botta SK, Singh A, Altenberg GA, Urbatsch IL. Directed evolution of P-glycoprotein cysteines reveals site-specific, non-conservative substitutions that preserve multidrug resistance. Biosci Rep. 2014 Jun 25;34(3). PMCID: PMC4069687 PubMed
- Ward AB, Szewczyk P, Grimard V, Lee CW, Martinez L, Doshi R, Caya A, Villaluz M, Pardon E, Cregger C, Swartz DJ, Falson PG, Urbatsch IL, Govaerts C, Steyaert J, Chang G. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13386-91. PMCID: PMC3746859. This journal has an impact factor of 9.8; the article has already been cited 53 times. PubMed
- Swartz DJ, Weber J, Urbatsch IL. P-glycoprotein is fully active after multiple tryptophan substitutions. Biochim Biophys Acta. 2013; 1828(3):1159-1168. PMCID: PMC3602414 PubMed
- Bai J, Swartz DJ, Protasevich II, Brouillette CG, Harrell PM, Hildebrandt E, Gasser B, Mattanovich D, Ward A, Chang G, Urbatsch IL. A gene optimization strategy that enhances production of fully functional P-glycoprotein in Pichia pastoris. PLoS One 2011; 6(8):e22577. PubMed
- Schölz C, Parcej D, Robenek H, Urbatsch IL, Tampé R. Transporter associated with antigen processing (TAP) is modulated by lipids. J. Biol. Chem. 2011; 286(15):13346-56. PubMed
- Johnson BJ, Lee JY, Pickert A, Urbatsch IL. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry 2010; 49(16):3403-11. PubMed
- Gutmann DAP, Ward A, Urbatsch IL, Chang G and van Veen HW. Understanding polyspecificity of multidrug ABC transporters: closing in on the gap in ABCB1. Trends Biochem Sci. 2010; 49(16):3403-11. PubMed
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, and Chang G. Structure of P-glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science 2009 Mar 27;323(5922):1718-22. PubMed
